In this printable students also use an equation to answer questions about elements and chemical reactions. A double replacement reaction will occur if a formation of a precipitate gas or water takes place.

Double Replacement Reaction Definition And Examples
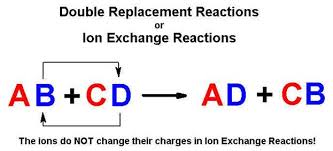
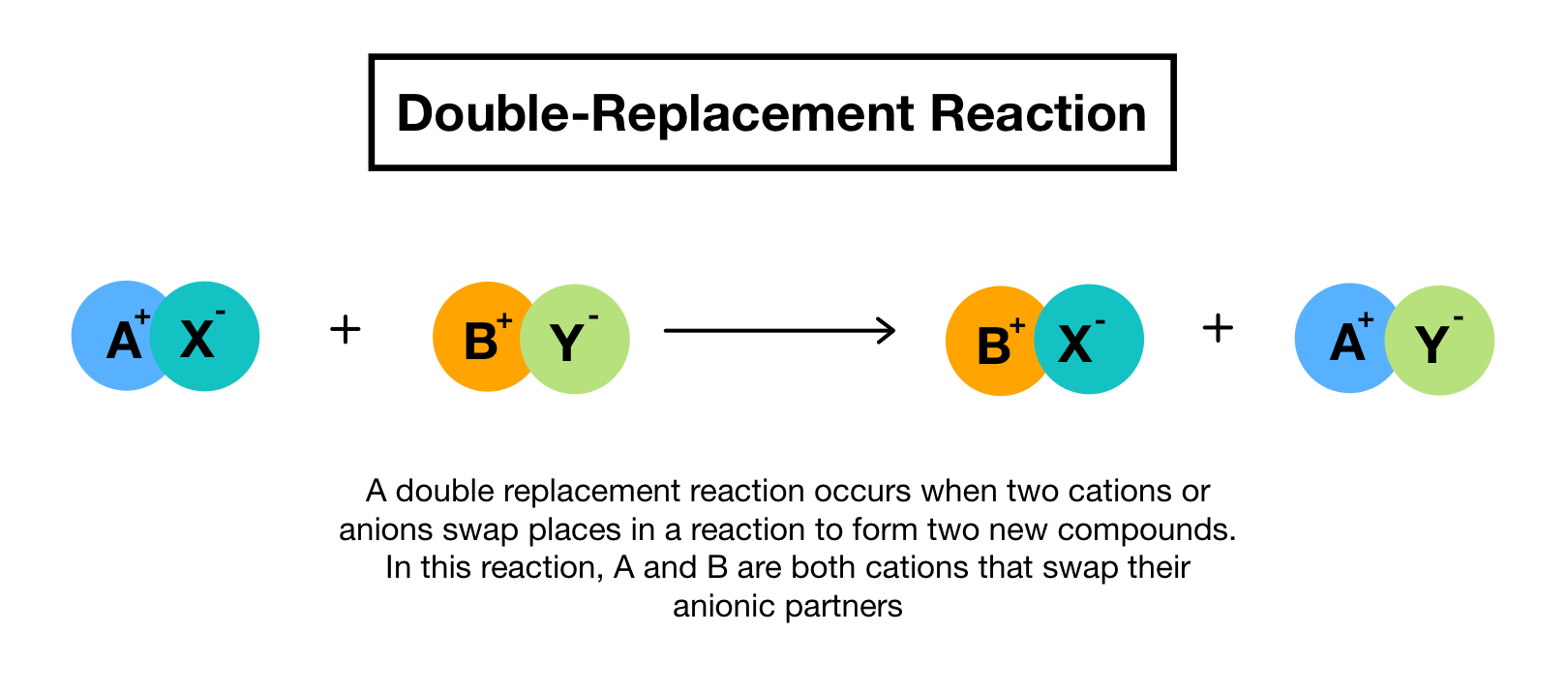
You can think of the reaction as swapping the cations or the anions but not swapping both since you would end up with the same substances you started with.

. Explain again that these are. A precipitation reaction is a double - replacement reaction in which one product is a solid precipitate. It isa chemical process involving the exchange of bonds between tworeacting chemicals and results in the creation of products withsimilar.
This is simply based on the solubility chart of inorganic compounds. AgNO₃aq NaClaq AgCls NaNO₃aq Agaq NO₃aq Naaq Claq AgCls Naaq NO₃aq This is a double replacement reaction because the silver ion and the sodium ion have exchanged partners. Zns 2 HClaq ZnCl2aq H2g 2 K 2H2O 2 KOH H2 note how the anion is written differently because we dont write water as.
Here are examples of single replacement reactions involving the cations. In double replacement reactions the positive ions exchange negative ion partners. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
This article will cover the main classifications of chemical reactions. Reactants Products Many of these reactions occur in an aqueous environment ie in a solution where ions and compounds. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
A double replacement reaction is represented by. The way I think of it since were dealing with ionic compounds is that when I write out a reaction I. Double replacement reactions are also called metathesis or double displacement reactions.
Solubility rules are used to predict whether some double-replacement reactions will occur. Because the replacement occurs in two places it is referred to as a double replacement. A single replacement reaction sometimes called a single displacement reaction is a reaction in which one element is substituted for another element in a compound.
Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. What Is a Double-Replacement Reaction. Double replacement reaction is a salt metathesis reaction.
Which of the following is an example of a single replacement reaction. A double-replacement reaction exchanges the cations or the anions of two ionic compounds. Double Replacement Reactions One of the main purposes of chemistry is to transform one set of chemicals the reactants into another set of chemicals the products via a chemical reaction.
AB CD AD CB Example. Synthesis reaction- a reaction that occurs when. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate.
When a replacement reaction occurs a new aqueous compound and. It is a reaction between two compounds such that each exchanges radicals with other. Some of the worksheets for this concept are work 5 double replacement reactions in these double replacement reactions work work 5 double replacement reactions chemistry replacement reaction work home types of chemical reactions predicting products of chemical reactions.
Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. Solubility rules are used to predict whether some double - replacement reactions will occur. Bottom of the cotton.
An aqueous compound formed from single replacement reaction An aqueous compound formed from double replacement reaction A solid formed from a double replacement reaction. Types of Chemical Reactions. Synthesis reaction decomposition reaction single replacement reaction and double replacement reactionWe also discuss what is a combustion reaction precipitation reaction and acid base reaction.
Double replacement reactions take the general form. The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound. Double replacement sometimes referred to as double displacement reactions are when parts of ionic compounds are switched to form two new ionic compounds.
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. Usually in these reactions when combining. Select two compounds above and this calculator will predict whether or not the reaction will occur in water.
The truefalse questions in this worksheet will help students review the process of a double-replacement reaction. A double displacement reaction also known as a double replacement reaction or metathesis is a type of chemical reaction where two compounds react and the positive ions cation and the negative. Double Replacement Reaction Worksheet.
Many double displacement reactions occur between ionic compounds that are dissolved in water. The solvent for a double replacement reaction is usually water and the reactants and products are usually ionic compoundsbut they can also be acids or bases. In this reaction one of the compounds formed is a precipitate which settles down at the.
In a double replacement reaction the reactants are always two compounds and the products are always two different compounds.

Double Replacement Double Displacement Reaction
What Is A Double Replacement Reaction In Chemistry Socratic
Double Replacement Reactions Definition Examples Expii

Introduction To Double Replacement Reactions Youtube

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

Double Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Single Replacement Reaction Definition And Examples

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
0 comments
Post a Comment